
- CHEMICAL EQUATION BALANCER PROGRAM HOW TO
- CHEMICAL EQUATION BALANCER PROGRAM .EXE
- CHEMICAL EQUATION BALANCER PROGRAM SOFTWARE
Computer analysis to produce univocal reactions sets: Possible application to the analysis of complex mixtures. International Journal of Mathematical Education in Science and Technology 1987, 745-751. Algebraic constructs for the graphical and computational solution to balancing chemical equations. Journal of the Chinese Chemical Society 2009, 56 A New Singular Matrix Method for Balancing Chemical Equations and Their Stability. Critical Reviews in Analytical Chemistry 2015, 45 “Why Not Stoichiometry” versus “Stoichiometry-Why Not?” Part I: General Context.


CHEMICAL EQUATION BALANCER PROGRAM SOFTWARE
Chemical Mass Calculator v.1.0 Small and fast software that calculates the molar mass of a chemical formula. Just enter the reactants and the products, and this tool will automatically add the.
CHEMICAL EQUATION BALANCER PROGRAM .EXE
exe program (originally python) to balance chemical equations. For example, if you double all of the coefficients, you still have a balanced equation: Chemical Equation Balancer v.1.0 This is a. The answer will appear below Always use the upper case for the first character in the element name and the lower case for the second character. Note: You could have written a balanced equation using multiples of the coefficients. Given the values x, y, p, q of a simple chemical equation of the type: The task is to find the values of constants b 1, b 2, b 3 such that the equation is balanced on both sides and it must be the reduced form. Instructions on balancing chemical equations: Enter an equation of a chemical reaction and click 'Balance'. This works! The balanced chemical equation is: If you try 3 O 2, then you have 6 oxygen atoms on the reactant side and also 6 oxygen atoms on the product side. How many atoms of aluminum are on each side of the following equation: 4Al + 3O 2 -> 2Al 2 O 3. When balancing equations a can be placed to the left of a formula of a substance to make the equations balanced. The large number to the left of a formula.
If you put a 2 in from of O 2, that will give you 4 atoms of oxygen, but you have 6 atoms of oxygen in the product (coefficient of 2 multiplied by the subscript of 3). The small number on the right of the chemical symbol.
CHEMICAL EQUATION BALANCER PROGRAM HOW TO
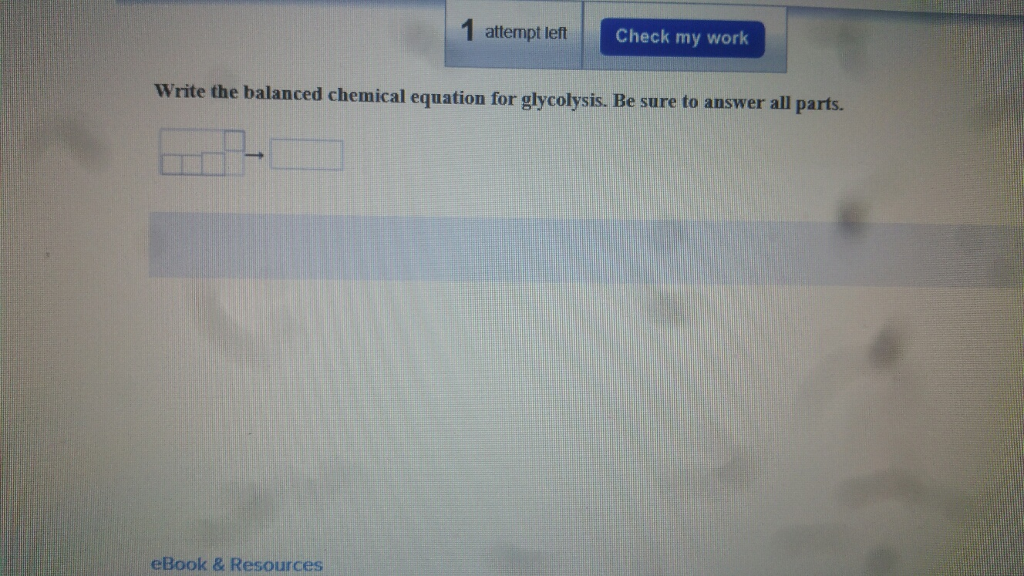
Then what is a balanced equation Let’s find out below and learn how to balance a chemical equation. Therefore, this is an unbalanced chemical equation. Now, look at the equation (use inspection) to see which coefficient will work to balance oxygen. Balanced Chemical Equations In the reaction Mg + O 2 MgO, the number of atoms of each element on either side of the arrow is not equal. The reason is that they usually appear in multiple reactants and products, so if you tackle them first you're usually making extra work for yourself. When balancing chemical equations, the last step is to add coefficients to oxygen and hydrogen atoms. Iron is balanced, with 4 atoms of iron on each side of the equation. This application can balance chemical equations, calculate molar masses and do some other stuff. By inspection (i.e., looking at it), you know you have to discard a coefficient of 2 for some higher number.ģ Fe doesn't work on the left because you can't put a coefficient in from of Fe 2O 3 that would balance it.Ĥ Fe works, if you then add a coefficient of 2 in front of the rust (iron oxide) molecule, making it 2 Fe 2O 3. A chemical equation is nothing but a written representation, using numbers and symbols, of the process that occurs during a chemical reaction. Chemical equations were first formulated in the year 1615 by a French chemist Jean Beguin.
While that would balance iron, you already know you're going to have to adjust oxygen, too, because it isn't balanced. A chemical equation is a common term of day to day chemistry. There is one atom of iron on the left and two on the right, so you might think putting 2 Fe on the left would work. Iron is present in one reactant and one product, so balance its atoms first.


 0 kommentar(er)
0 kommentar(er)
